Miljöpåverkan
Isofluran
Miljörisk:
Användning av isofluran har bedömts medföra försumbar risk för miljöpåverkan.
Nedbrytning:
Isofluran är potentiellt persistent.
Bioackumulering:
Isofluran har låg potential att bioackumuleras.
Läs mer
Detaljerad miljöinformation
Detailed background information
Due to the prescribed application regime of the medicine and its physical-chemical properties, the parent compound isoflurane (CAS 26675-46-7) is mainly emitted to the air compartment. Indeed, a minor amount of isoflurane and its metabolites fluoride and trifluoroacetic acid (TFA) are maybe emitted down-the-drain. Only the latter releases, parent and metabolite, have been examined in the following risk classification. Thus, only the potential risk to the aquatic compartment is addressed here.
Environmental Risk Classification
Predicted Environmental Concentration (PEC): parent compound
PEC is calculated according to the following formula:
PEC (μg/L) = (A*109*(100-R))/(365*P*V*D*100) = 1.37*10-6*A(100-R)
PEC = 0.000025 μg/L
Where:
A = 185.4 kg * 0.1% (total sold amount API in Sweden year 2022, data from IQVIA (2023); reduced by fraction excreted). The 0.1% represents a worst-case assumption; Justification on reduction sees metabolism data below.
R = removal rate (due to loss by adsorption to sludge particles, by volatilization, hydrolysis or biodegradation) = 0 % This value represents a worst-case assumption due to volatilisation; Justification sees data below.
P = number of inhabitants in Sweden = 10*106
V (L/day) = volume of wastewater per capita and day = 200 (ECHA default) (ECHA, 2016)
D = factor for dilution of waste water by surface water flow = 10 (ECHA default) (ECHA, 2016)
Predicted No Effect Concentration (PNEC): parent compound
Ecotoxicological studies
For isoflurane information on aquatic toxicity is not available
Algae:
Not available
Crustacean:
Acute toxicity
Not available
Chronic toxicity
Not available
Fish:
Acute toxicity
Not available
Chronic toxicity
Not available
Environmental risk classification (PEC/PNEC ratio): parent compound
Based on the fact that for isoflurane information on aquatic toxicity is not available, the phrase «Risk of environmental impact of isoflurane cannot be excluded, since no ecotoxicity data are available» has to be chosen.
However, according to the European Medicines Agency guideline on environmental risk assessment of medicinal products (EMA/CHMP/SWP/4447/00), use of isoflurane is unlikely to represent a risk for the environment, because the predicted environmental concentration (PEC) is below the action limit 0.01 μg/L.
Moreover, the available information for the metabolite TFA (see below), justifies the phrase «Use of isoflurane has been considered to result in insignificant risk.»
Therefore, the use of isoflurane under the prescribed application regime is unlikely to represent a risk for the environment, although, due to the missing of experimentally determined ecotoxicity data for the parent compound, an environmental impact of isoflurane cannot be completely ruled out.
Overall, the distinct difference to the action limit for the parent compound as well as the low risk characterization ratio for the metabolite justifies the phrase «Use of isoflurane has been considered to result in insignificant risk.»
Predicted Environmental Concentration (PEC): metabolite TFA
PEC is calculated according to the following formula:
PEC (μg/L) = (A*109*(100-R))/(365*P*V*D*100) = 1.37*10-6*A(100-R)
PEC = 0.000031 μg/L
Where:
A = 185.4 kg * 114.02 g/mol / 184.49 g/mol * 0.2% (total sold amount API in Sweden year 2017, data from IQVIA (2018); reduced by fraction excreted). The 0.2% represents a worst-case assumption; Justification on reduction see metabolism data below.
R = removal rate (due to loss by adsorption to sludge particles, by volatilization, hydrolysis or biodegradation) = 0 %
P = number of inhabitants in Sweden = 10*106
V (L/day) = volume of wastewater per capita and day = 200 (ECHA default) (ECHA, 2016)
D = factor for dilution of waste water by surface water flow = 10 (ECHA default) (ECHA, 2016)
Predicted No Effect Concentration (PNEC): metabolite TFA
Ecotoxicological studies
Review of effects of TFA on environmental organisms is published by Solomon et al. (2016), which cites effect values based on biomass given by Berends et al (1999). A registration dossier under REACh is available on the ECHA website (ECHA, 2018) describing several aquatic toxicity studies with TFA (studies performed with sodium trifluoroacetate; concentrations re-calculated for TFA). It should be noted that the original study reports could not be evaluated. Instead, only information publicly disseminated on the ECHA website served as basis of this assessment. Therefore, definitive quality and reliability cannot be assessed from this limited information.
However, the registrants regarded the studies as reliable.
Algae:
Green alga (Pseudokirchneriella subcapita (synonym Raphidocelis subcapitata) formerly known as Selenastrum capricornutum) (OECD 201, GLP, including analytical monitoring) (ECHA, 2018)
EC50 72 h (growth rate) = 237 mg/L
EC10 72 h (growth rate) = 5.6 mg/L
Marine alga (Phaeodactylum tricornutum) (OECD 201, GLP, no analytical monitoring, effect values based on nominal concentrations) (ECHA, 2018)
EC50 96 h (growth rate) > 97 mg/L
NOEC 96 h (growth rate) > 97 mg/L
Blue green alga (Anabaena flos-aquae) (US-EPA 540/09-82-020, GLP, including analytical monitoring, effect values based on nominal concentrations) (Smyth et al., 1994)
EC50 120 h (growth rate) > 1997 mg/L
NOEC 120 h (growth rate) = 499 mg/L
Crustacean (Daphnia magna):
Acute toxicity
EC50 48 h (immobility) > 999 mg/L (nominal concentration, OECD 202, GLP, limit test with 1200 mg/L sodium trifluoroacetate, including analytical monitoring)
Chronic toxicity
NOEC 21 days (reproduction rate, survival of adults) > 25 mg/L (nominal concentration, OECD 211, GLP, including analytical monitoring, no effect observed at highest concentration tested)
Fish:
Acute toxicity
Zebra fish (Danio rerio) LC50 96 h > 999 mg/L (nominal concentration, OECD 203, GLP, limit test with 1200 mg/L sodium trifluoroacetate, including analytical monitoring)
Chronic toxicity
No experimental results available
According to REACh Technical Guidance Document, Chapter R.10 (ECHA, 2008) an assessment factor of 50 applies to the lowest of two long term results (e.g. EC10 or NOECs) representing two trophic levels when such results have been generated covering that level showing the lowest L(E)C50 in the short-term tests. NOEC of the most recent study with the green alga Pseudokirchneriella subcapita has been used for this calculation since it is the most sensitive of the three tested species and the most reliable study. This PNEC is regarded a worst case estimate. For comparison, the REACh dossier derives a PNEC of 0.56 mg/L based on the EC10 of 5.6 mg/ L and using an AF of 10.
PNECsurface water = lowest NOEC/50 = 112 µg/L
Environmental risk classification (PEC/PNEC ratio): metabolite TFA
According to the European Medicines Agency guideline on environmental risk assessment of medicinal products (EMA/CHMP/SWP/4447/00), TFA as a relevant metabolite from the use of isoflurane is unlikely to represent a risk for the environment, because the predicted environmental concentration (PEC) is below the action limit 0.01 μg/L.
Moreover, based on the available information for the metabolite TFA,
PEC/PNEC = 0.000031/112 = 0.00000028, i.e. PEC/PNEC ≤ 0.1 which justifies the phrase «Use of isoflurane has been considered to result in insignificant risk.»
Degradation
Biotic degradation
Ready degradability:
For isoflurane studies on ready biodegradability are not available.
For the metabolite TFA biodegradation studies are presented in the REACh dossier (ECHA, 2012):
Test result: 0 % degradation within 28 days (OECD 301D).
These results indicate that isoflurane as well as TFA can be regarded as not readily biodegradable.
Inherent degradability:
Information on inherent biodegradability of isoflurane is not available.
For the metabolite TFA a modified SCAS Test is presented in the REACh dossier (ECHA, 2012) indicating that TFA is not biodegraded.
Simulation studies:
STP simulation studies and test results in water, sediment and total system are not available.
TFA was shown to be persistent in water compartments since it was not biodegraded during a year-long study using laboratory aquatic microcosms and ecosystem sediment-water systems (Ellis et al., 2001).
Abiotic degradation
Hydrolysis:
Information on hydrolysis is not available.
Photolysis:
Information on photolysis in water is not available.
Justification if R is not equal to 0, e.g. modelling results using SimpleTreat:
As isoflurane is not readily biodegradable, and simulation studies are not available, the default value was used for removal rate R = 0. However, this is considered to represent a worst-case approach for isoflurane, as modelling results using SimpleTreat suggest that within STP about 91.5% is emitted to air, 1.11% to sludge, and only 7.43% to water.
As the metabolite TFA is not readily biodegradable, simulation studies are not available, and microcosms/field studies suggest persistence in the environment, the default value was used for removal rate R = 0. Modelling results using SimpleTreat suggest that within STP about 0.01% is emitted to air, 0.238% to sludge, and 99.8% to water.
Justification of chosen degradation phrase:
Based on the information that isoflurane as well as its metabolite TFA are not readily biodegradable, and reliable simulation studies are not available, the phrase “isoflurane is potentially persistent” is thus chosen.
Photodegradation:
In the atmosphere isoflurane could be removed by chemical reaction with radicals, by photolysis and by wet or dry deposition. The degradation time is assumed to be limited by the reaction with the hydroxyl radical (OH•). The following rate coefficients kOH are described:
McLoughlin et al. (1993) < 3E-13 cm3/molecule-sec at 300 K
EpiSuite (estimation): 2.33E-14 cm3/molecule-sec at 298 K
Brown et al. (1989) 2.1E-14 cm3/molecule-sec (at 300 K)
Beach et al. (2001) 1.9E-14cm3/molecule-sec at 293 K
Langbein et al. (1999): 1.7E-14 cm3/molecule-sec at 298 K
Sulbaek Andersen et al. (2012) 1.5E-14 cm3/molecule-sec at 296 K
Sulbaek Andersen et al. (2010) 1.01E-14 cm3/molecule-sec at 272 K
The life-times given in the literature are between 2 and 6 years depending on the rate constant, OH radical concentration, and temperature.
For TFA the estimated rate coefficient kOH is 0.52E-012 cm3/molecule-sec at 298 K corresponding to a half-life of 31 d (EpiSuite, AOPWIN). The major elimination pathway of TFA in air is rainout.
Adsorption and desorption to soil
The soil adsorption coefficient (Koc) of isoflurane was calculated by ACD/Labs to be 338 L/kg. Estimations via EpiSuite show Koc values of 97.54 L/kg (MCI method) and 94.18 L/kg. (Kow method with a logKow of 2.06). Using the logPow and the recommended QSAR of the TGD for non-hydrophobics, results in a Koc of 123 L/kg. Thus, adsorption of isoflurane to soil and sediment is assumed to be low.
For TFA the soil adsorption coefficient (Koc) was estimated via EpiSuite. This estimation results in values of 3.231 L/kg (MCI method) and 2.706 L/kg. (Kow method with a logKow of 0.5). Using the logPow and the recommended QSAR of the TGD for non-hydrophobics, results in a Koc of 19.1 L/kg. However, the logPow and the Koc of this structure may be sensitive to pH, and thus may vary significantly. In a screening test presented in the REACh dossier (ECHA, 2012), no adsorption to three different standard soils was observed. In addition, in soil retention studies on a total of 54 soil samples the Kd were ranged between 0.17 to 20 L/kg for all soil location (Richey et al., 1997). The results indicate that retention of TFA by soil surfaces is dependent upon pH, soil organic matter and mineral surfaces and the presence of other anions in soil solution. Overall, TFA can be considered as a mobile organic compound in the majority of soils.
Volatilisation
Distribution of isoflurane between air and water (Henry’s law constant) was estimated from the ratio of the vapour pressure to the water solubility. The calculated value of 2950 Pa m3/mole at 25 °C, resulting in an air-water partitioning coefficient of 0.596 at 12°C, indicates a rapid and significant volatilization from water.
For the metabolite TFA experimental Henrys law constants are presented in the REACh dossier (ECHA, 2012): the values ranges from 5800 mol/dm3/atm (Kutsuna and Hori, 2008) and 8950 mol/dm3/atm (Bowden et al., 1996) corresponding to a value of 0.00713 and 0.0112 Pa m3/mol at 25 °C, respectively. This indicates a very low volatility potential of the metabolite.
Bioaccumulation
Bioconcentration factor (BCF):
Bioconcentration study for isoflurane is not available.
As well, an experimental bioconcentration study for the metabolite TFA is not available, that would enable the derivation of a reliable BCF. However, results on the incorporation of TFA into freshwater sediment organisms as well as estimation data (EpiSuite/BCFBAF) indicate only an insignificant potential for bioconcentration in aquatic species.
Partitioning coefficient:
An experimental study according to OECD 107 on the logPow of isoflurane determined a logPow > 1.9 (Baxter, 2012). With the analytical method performed in this study it was not possible to detect isoflurane in the water phase, thus no exact value could be determined. However, this experimentally determined lower limit of the logPow is similar to the calculated values of 2.12 at 25°C (ACD/Labs) and 1.51 (EpiSuite/KOWWIN v1.67). Moreover, these values are confirmed by the generally accepted experimental value of 2.06 given by Hansch et al (1995).
Isoflurane: Log Dow = 2.06 at pH 7
For TFA estimated values for logPow of 0.5 (EpiSuite/KOWWIN v1.68) and 1.35 ± 0.38 (ACD/Labs) could be calculated. However, the partitioning is pH dependent as TFA dissociates in water and is expected to exist almost entirely in the anion form (dissociation constant pKa = 0.52); pH-dependence could be calculated using ACD/Labs.
TFA: estimated Log Dow = - 2.4 at pH 7
Justification of chosen bioaccumulation phrase:
Since log Dow < 4 at pH 7, isoflurane has low potential for bioaccumulation. Moreover, toxicokinetic data from humans show that isoflurane does not remain in human bodies but is released back into the air.
Since log Dow < 4 at pH 7, TFA has low potential for bioaccumulation.
Excretion (metabolism)
Isoflurane predominantly leaves the human body via pulmonary exhalation and reaches the atmosphere as the main target compartment. Scientific literature demonstrates that the administered isoflurane is mainly emitted unchanged into the atmosphere. Only a minor fraction of fluranes is excreted unchanged in urine (Saber and Sorig Hougaard, 2009).
The metabolism of isoflurane has been comprehensively reviewed (Kenna and van Pelt, 1994; Saber and Sorig Hougaard, 2009). Isoflurane is metabolized to the stable metabolites fluoride, chloride and trifluoroacetic acid (TFA), which are excreted via urine. It was determined that the postoperative increase of urinary excretion of fluoride and organic fluorine accounted for less than 0.2% (Holaday et al., 1975), which is in alignment to SPC Sweden.
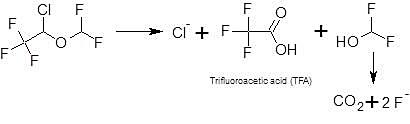
The portion of organic fluorine is < 0.1% (Holaday and Fiserova-Bergerova, 1979). Thus into wastewater, isoflurane is excreted assuming all organic fluorine is unchanged parent – as a worst-case assumption - to < 0.1% as parent compound and to 0.2% as metabolite trifluoroacetic acid (TFA). The pharmacological activity of the metabolite is not known.
A reduction of A (total sold amount API in Sweden 2017) in the PEC calculation is justified based on excretion/metabolism as follows:
Isoflurane (Parent):
A = 185.4 kg * 0.1%
TFA (Metabolite):
A = 185.4 kg * MW(metabolite)/MW(parent) * M
= 185.4 kg * 114.02 g/mol / 184.49 g/mol * 0.2%
PBT/vPvB assessment
As both Isoflurane and its metabolite TFA have low potential for bioaccumulation, they do not fulfil the criteria for PBT and/or vBvP substances and thus should not be flagged.
According to the established EU criteria, the medicine should not be regarded as a PBT/vPvB substance.
References
Baxter, 2012. Experimental study report: Isoflurane, Partition Coefficient n-Octanol/Water (OECD 107), Shake Flask Method. Performed by Siemens AG, Prozess-Sicherheit, Industriepark Höchst, B 596 & B 598, 65926 Frankfurt am Main, Germany.
Beach SD, Hickson KM, Smith IWM and Tuckett RP, 2001. Rate constants and Arrhenius parameters for the reactions of OH radicals and Cl atoms with CF3CH2OCHF2, CF3CHClOCHF2 and CF3CH2OCClF2, using the discharge-flow/resonance fluorescence method. Physical Chemistry Chemical Physics, 3, 3064-3069.
Berends AG, Boutonnet JC, De Rooij CG, and Thompson RS, 1999. Toxicity of trifluoroacetate to aquatic organisms. Environmental Toxicology and Chemistry. 18:1053–1059
Bowden DJ, Clegg SL and Brimblecombe P, 1996. The Henry's law constant of trifluoroacetic acid and its partitioning into liquid water in the atmosphere. Chemosphere, 32, 405-420
Brown AC, Canosa-Mas CE, Parr AD, Pierce JM and Wayne RP, 1989. Tropospheric lifetimes of halogenated anaesthetics. Nature, 341, 635-637.
ECHA, European Chemicals Agency, 2008. Guidance on information requirements and chemical safety assessment. https://echa.europa.eu/documents/10162/13632/information_requirements_r10_en.pdf/bb902be7-a503-4ab7-9036-d866b8ddce69
ECHA, European Chemicals Agency, 2016. Guidance on information requirements and chemical safety assessment chapter R.16: Environmental exposure assessment version 3.0 February 2016. https://echa.europa.eu/documents/10162/13632/information_requirements_r16_en.pdf/b9f0f406-ff5f-4315-908e-e5f83115d6af
ECHA, European Chemicals Agency, 2018. REACH-Registration dossier trifluoroacetic acid (CAS 76-05-1), last modified 2012-06-18. European Chemicals Agency, Helsinki, Finland. Publicly available under: https://www.echa.europa.eu/web/guest/registration-dossier/-/registered-dossier/5203
Ellis DA, Hanson ML, Sibley PK, Shahid T, Fineberg NA, Solomon KR, Muir DC and Mabury SA, 2001. The fate and persistence of trifluoroacetic and chloroacetic acids in pond waters. Chemosphere, 42, 309-318.
Hansch C, Leo A, Hoekman D, 1995. Exploring QSAR. Hydrophobic, electronic, and steric constants. American Chemical Society, Washington, DC (USA)
Holaday DA, Fiserova-Bergerova V, Latto IP, Zumbiel MA, 1975. Resistance of isoflurane to biotransformation in man. Anesthesiology 43: 325-32.
Holaday, DA, Fiserova-Bergerova V, 1979. Fate of fluorinated metabolites of inhalation anesthetics in man. Drug Metab Rev 9(1): 61-78.
IQVIA, 2023. Consumption assessment in kg for input to enviromental classification - updated 2023 (data 2022), Project 1048212
Kenna JG and van Pelt FNAM, 1994. The metabolism and toxicity of inhaled anaesthetic agents. Anaesthetic Pharmacology Review, 2, 29-42
Kutsuna S and Hori H, 2008. Experimental determination of Henry's law constants of trifluoroacetic acid at 278–298 K. Atmospheric Environment, 42, 1399-1412
Langbein T, Sonntag H, Trapp D, Hoffmann A, Malms W, Roth EP, Mors V and Zellner R, 1999. Volatile anaesthetics and the atmosphere: atmospheric lifetimes and atmospheric effects of halothane, enflurane, isoflurane, desflurane and sevoflurane. British Journal of Anaesthesia, 82, 66-73
McLoughlin P, Kane R and Shanahan I, 1993. A Relative Rate Study of the Reaction of Chlorine Atoms (Cl) and Hydroxyl Radicals (Oh) with a Series of Ethers. International Journal of Chemical Kinetics, 25, 137-149
Richey DG, Driscoll CT and Likens GE, 1997. Soil Retention of Trifluoroacetate. Environ Sci Technol, 31, 1723-1727
Saber AT and Sorig Hougaard K, 2009. 141. Isoflurane, sevoflurane and desflurane. Arbete och Hälsa, 43
Smyth DV, Thompson R S, E Gillings, 1994. Sodium Trifluoroacetate: Toxicity to the Blue-green Alga, Anabaena Flos-aquae, and to the Freshwater Diatom, Navicula Pelliculosa, with Cover Letter dated 06/27/94. Brixham Environmental Lab., NTIS report: OTS0557458.
Solomon KR, Velders GJM, Wilson SR, Madronich S, Longstreth J, Aucamp PJ, Bornman JF. 2016. Sources, fates, toxicity, and risks of trifluoroacetic acid and its salts: Relevance to substances regulated under the montreal and kyoto protocols. Journal of Toxicology and Environmental Health - Part B: Critical Reviews. 19(7):289-304
Sulbaek Andersen MP, Nielsen OJ, Karpichev B, Wallington TJ and Sander SP, 2012. Atmospheric Chemistry of Isoflurane, Desflurane, and Sevoflurane: Kinetics and Mechanisms of Reactions with Chlorine Atoms and OH Radicals and Global Warming Potentials. J Phys Chem A. 116(24):5806-5820
Sulbaek Andersen MP, Sander SP, Nielsen OJ, Wagner DS, Sanford TJ, Jr. and Wallington TJ, 2010. Inhalation anaesthetics and climate change. British Journal of Anaesthesia, 105, 760-766.





